3 Band gap energy and band edge positions of different semiconductor... Download Scientific
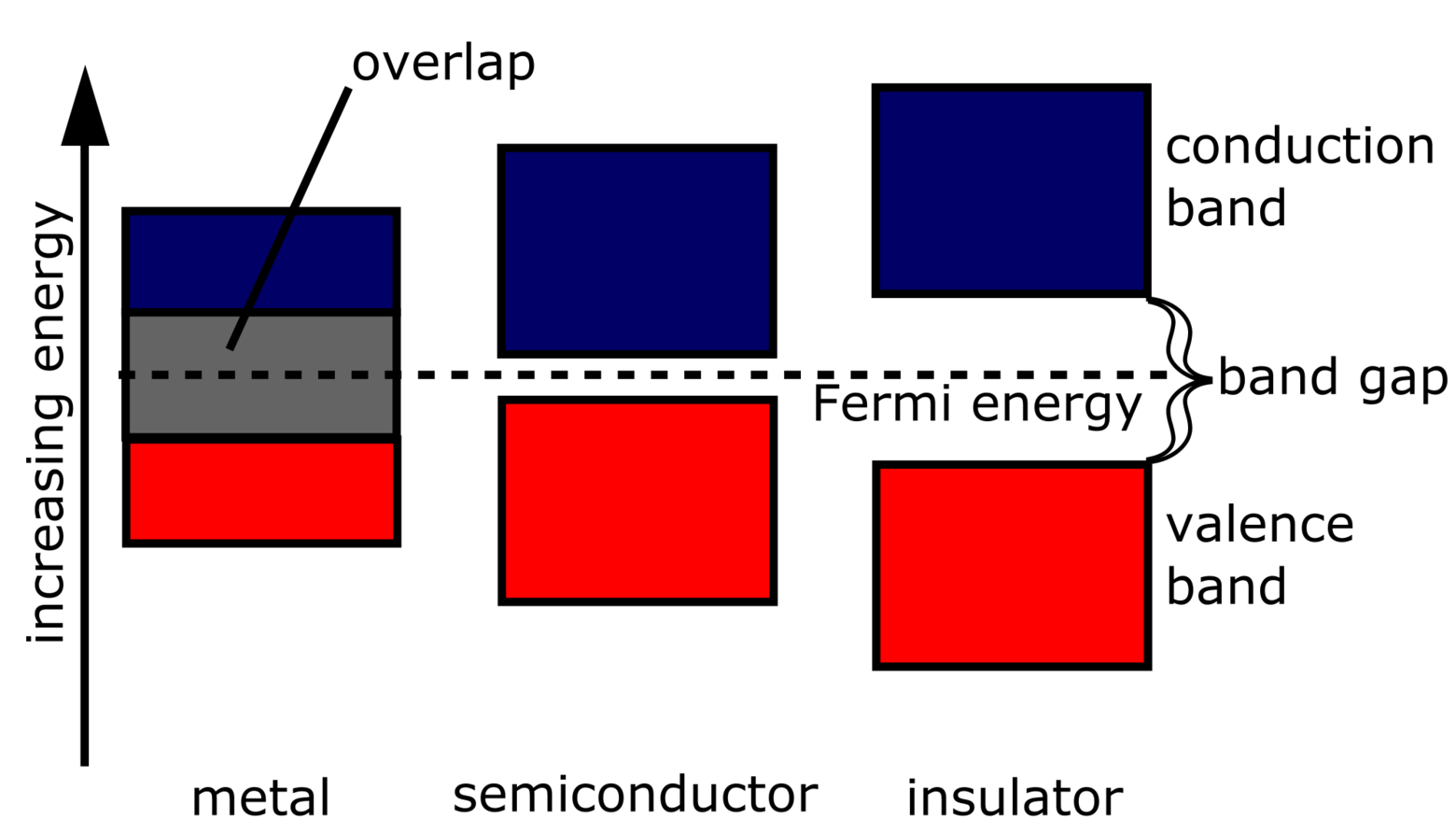
The energy gap in the insulator is very high up to 7eV. The material cannot conduct because the movement of the electrons from the valence band to the conduction band is not possible.. The energy band diagram of semiconductors is shown where the conduction band is empty and the valence band is completely filled but the forbidden gap between.

(Figure 4 from [39]). Energy gap diagram of the threeregion model... Download Scientific Diagram
How does the band gap energy vary with composition?There are two important trends (1) Going down a group in the periodic table, the gap decreases:. C (diamond) > Si > Ge > α-Sn. E gap (eV): 5.4 1.1 0.7 0.0. This trend can be understood by recalling that E gap is related to the energy splitting between bonding and antibonding orbitals.This difference decreases (and bonds become weaker) as the.
6 Energy band structures of GaAs and silicon as in [5]. A... Download Scientific Diagram
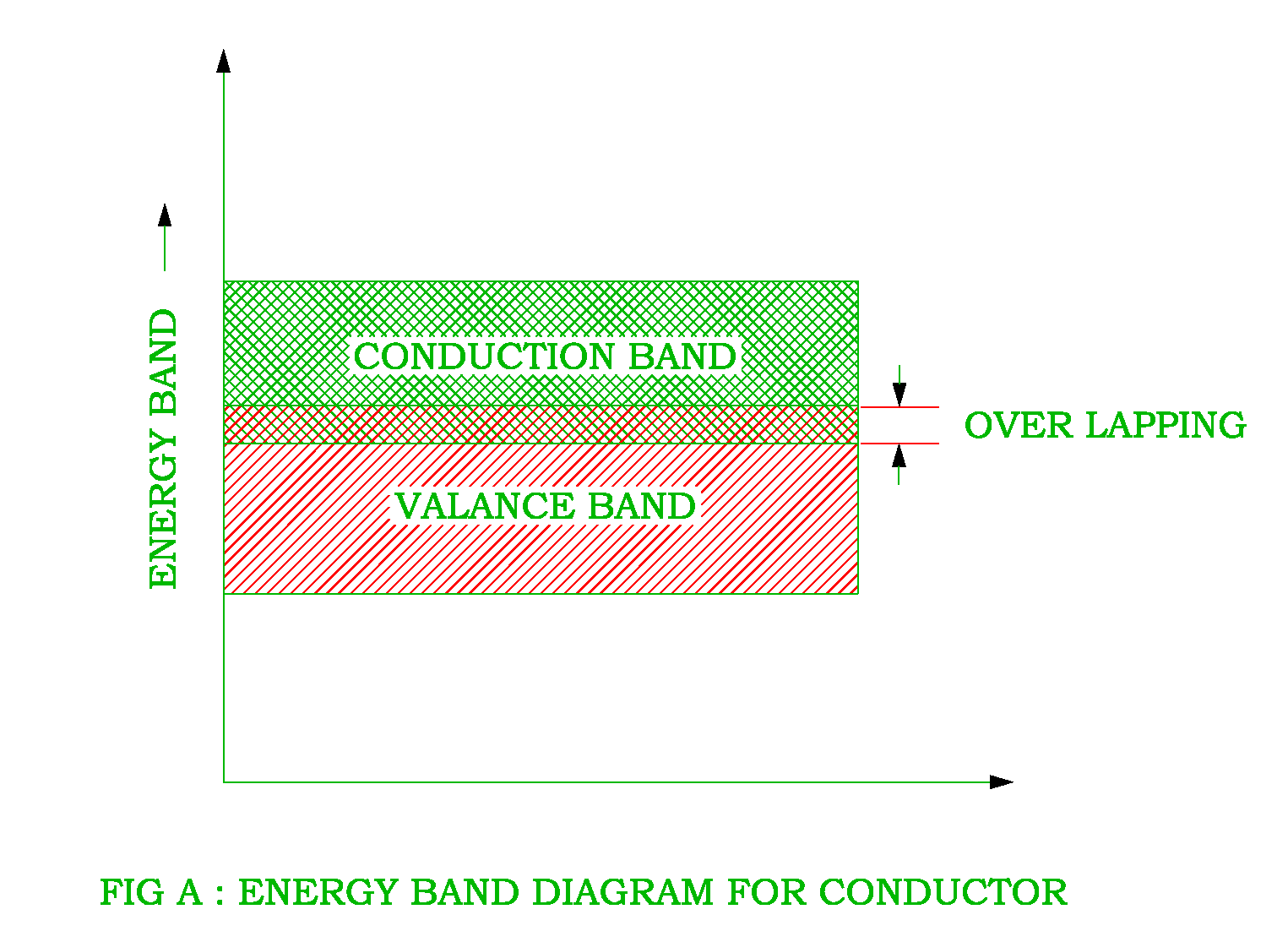
The energy band gap is the energy difference between a material's valence and conduction bands. Conductors have overlapping valence and conduction bands, allowing electrons to move quickly through the material and conduct electricity. Examples of conductors include copper (Cu), aluminum (Al), and silver (Ag).
band gap diagram electric field
The band gap (E G) is the gap in energy between the bound state and the free state, between the valence band and conduction band. Therefore, the band gap is the minimum change in energy required to excite the electron so that it can participate in conduction. Schematic of the energy bands for electrons in a solid.

1. (a) Energy band diagram of AlGaN/GaN HEMT illustrating band gap... Download Scientific Diagram
statistically, a few electrons gain enough energy to hop across the gap and conduct. Finally, in an insulator, the space between the highest full band and the lowest energy band is very large: it takes so much energy for an electron to jump to the conduction band that it doesn't happen under normal temperature and operating conditions.
Energy band diagram demonstrating different band gap energies. Download Scientific Diagram
Figure 9.6.2 9.6. 2: The dependence of energy-level splitting on the average distance between (a) two atoms, (b) four atoms, and (c) a large number of atoms. For a large number of electrons, a continuous band of energies is produced. Energy bands differ in the number of electrons they hold. In the 1 s and 2 s energy bands, each energy level.

Energy band diagram for perovskite solar cell on a scaffold structure... Download Scientific
The anticrossing diagram shows that rather counter-intuitively, even a weak periodic potential changes the topology of the initially parabolic dispersion relation radically, connecting its different branches, and thus creating the energy gaps.. We see that now at small \(\beta\) the first energy gap grows much faster than the higher ones: \.

photochemistry What is the explanation of the energy gap law in radiationless transitions
The separation between the conduction band and valence band on the energy band diagram is known as the forbidden energy gap (band-gap, E g ). The width of the energy gap is a measure of the bondage of valence electrons to the atom. The greater the energy gap, the more tightly the valence electrons are bound to the nucleus.
Energy Band Gap Simulation
1-1. Energy band diagram. 1-1. Energy band diagram. Free electrons in a material allow a free flow of electricity. Although being part of atoms, free electrons are so loosely bound to atoms in a material, they can move about freely. In classical physics, the Bohr model is a physical model that consists of a small atomic nucleus of protons and.

Bandgap energetics diagram of (a) ZnO and (b) ZnOgraphene or ZnOCNT... Download Scientific
Direct band gap: the lowest-energy state above the band gap has the same k as the highest-energy state beneath the band gap.. When the horizontal lines in these diagram are slanted then the energy of the level or band changes with distance. Diagrammatically, this depicts the presence of an electric field within the crystal system..
Top Variations of (a) energy gaps and (b) relative energies among... Download Scientific Diagram
The energy band diagram of a quantum well is shown in Fig. 1.3a, drawn assuming that the band-bending adjacent to the interfaces occurs over distances much larger than the width of the well and barriers and can be ignored on this scale. The depths of the conduction and valence band wells are determined by the heterostructure band offsets ΔE c, ΔE v which sum to the band gap difference at the.

(a) Energy gap versus temperature of a NbN sample for both main axes of... Download Scientific
An extension of the simple band energy diagram with only the vertical axis labelled as energy, with the horizontal axis unlabelled, is to plot the energy vertically against wave vector, k. From de Broglie's relationship p = hk where p is momentum and h is Planck's constant, h, divided by 2 π. Such plots therefore relate energy to momentum.

Energy gap of graphene. (a) The schematic diagram of band dispersion at... Download Scientific
In a direct band gap semiconductor, the top of the valence band and the bottom of the conduction band occur at the same value of momentum, as in the schematic below. In an indirect band gap semiconductor, the maximum energy of the valence band occurs at a different value of momentum to the minimum in the conduction band energy: The difference.
Band gap Energy Education
In solid-state physics and solid-state chemistry, a band gap, also called a bandgap or energy gap, is an energy range in a solid where no electronic states exist. In graphs of the electronic band structure of solids, the band gap refers to the energy difference (often expressed in electronvolts ) between the top of the valence band and the bottom of the conduction band in insulators and.

2 Energy bandgap diagram Download Scientific Diagram
A band gap is the distance between the valence band of electrons and the conduction band. Essentially, the band gap represents the minimum energy that is required to excite an electron up to a state in the conduction band where it can participate in conduction. [1] The lower energy level is the valence band, and thus if a gap exists between.

Four energy gap functions depending on the 'Te' concentration. Download Scientific Diagram
Semiconductors are defined by their name: they are kinda conductive. These materials have a band gap, but it's not as big as that of an insulator. Often in the field, 3 eV 3 e V serves as a rough cut-off: band gaps below this energy belong to semiconductors, while higher energy systems are considered insulating.